How many milliliters of 12.0 M HCl(aq) must be diluted with water to make exactly 500. mL of 3.00 M hydrochloric acid? | Socratic
If 250ml of 1M HCl is diluted to 1000ml, what would be molarity of the diluted solution? What will be the pH? - Quora
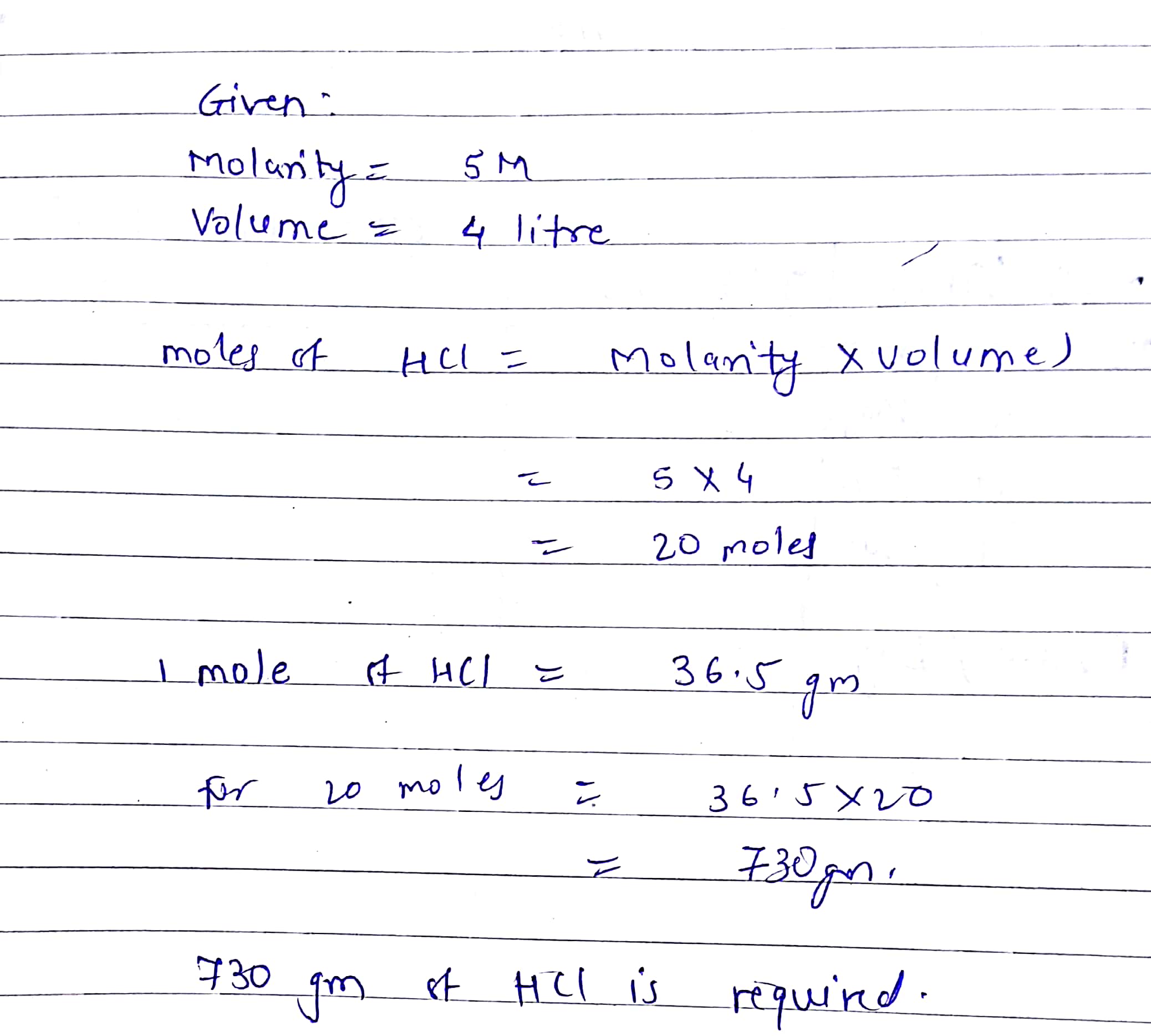
How many grams of hydrogen chloride,HCL are required to prepare 4 Litre of 5M HCL in water - 2ilx7jll

Discussion | 31 5. How many grams are needed to make 2.5L of 1.5M HCl solution? CI 350 レ. 6. How many grams are needed to make 500...

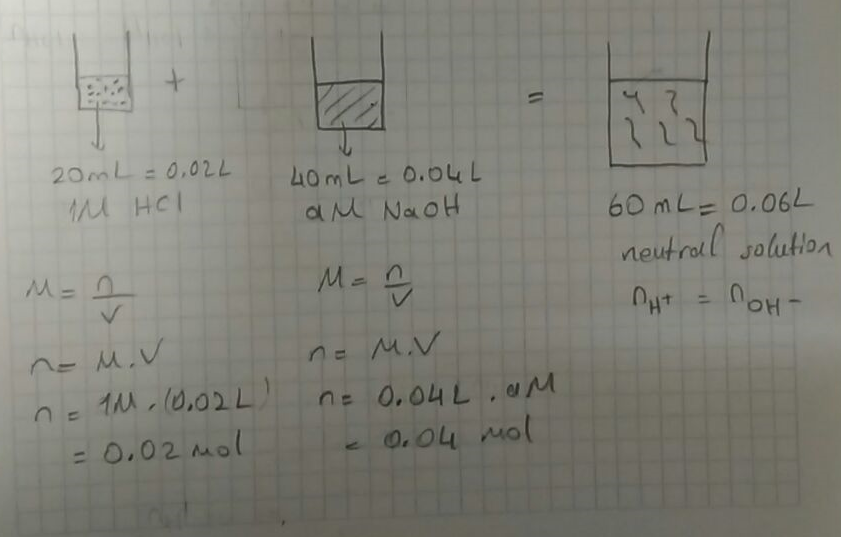
1. Which Vhich will basic buffer? 100 mL of 0.1 M HCl + 100 ml of 0 1 (a) 100 mL of o M NaOH (b) 50 mL of 0.1 M M

SOLVED: I would dilute 100.0 mL of a 5.0 M HCl solution to make a 0.20 M HCl solution. What will be the final volume? (2 pts) 10. Calculate the molarity of

A 37%(w/w) solution of Hydrochloric acid has a density of 1 18 g/mol What volume of this solution should - Chemistry - Some Basic Concepts of Chemistry - 13482901 | Meritnation.com
![BT021] 1M Tris-HCl, pH 8.5 | Biosolution BT021] 1M Tris-HCl, pH 8.5 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2015/05/BT016-1M-Tris-HCl.jpg)






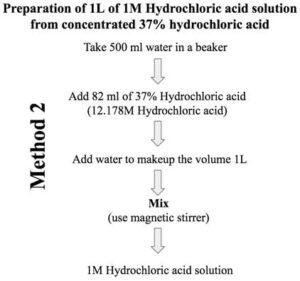

![ANSWERED] In lab, students are given 100.0 mL of 1M... - Physical Chemistry - Kunduz ANSWERED] In lab, students are given 100.0 mL of 1M... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question/raw/74831241-1659788608.1231616.jpeg)



